A 47 year old woman with NVD and NVE due to PDR returned 2 months after PRP. Angiography confirming diffuse macular leakage consistent with clinically significant macular edema (CSME) possibly exacerbated by the PRP, and progressive NVD and NVE.
Intravitreal bevacizumab (1.25 mg) was administered intravitreally.
When she returned 8 weeks later, angiography confirmed that her macular edema resolved and NV regressed.
-
 Early Phase PDR Pre-Treatment
Early Phase PDR Pre-Treatment
Aug 10 2014 by Thomas A. Ciulla, MD, MBA, FASRS
Note the active NVD and NVE.
Condition/keywords: intravitreal bevacizumab, proliferative diabetic retinopathy (PDR)
-
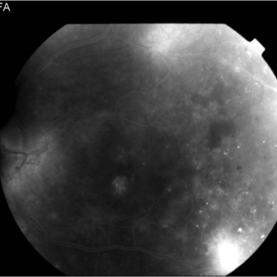 Late Phase PDR Pre-Treatment
Late Phase PDR Pre-Treatment
Aug 10 2014 by Thomas A. Ciulla, MD, MBA, FASRS
The later frames of the angiogram reveal NVD, NVE, and macular leakage temporally.
Condition/keywords: intravitreal bevacizumab, proliferative diabetic retinopathy (PDR)
-
 Early Phase PDR Post-Treatment
Early Phase PDR Post-Treatment
Aug 10 2014 by Thomas A. Ciulla, MD, MBA, FASRS
Intravitreal bevacizumab (1.25 mg) was administered intravitreally. When she returned 8 weeks later, her macular edema resolved and NV regressed.
Condition/keywords: intravitreal bevacizumab, proliferative diabetic retinopathy (PDR)
-
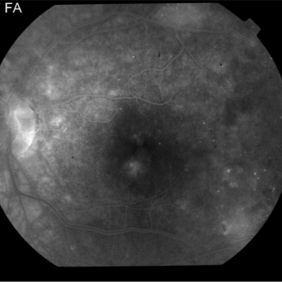 Late Phase PDR Post-Treatment
Late Phase PDR Post-Treatment
Aug 10 2014 by Thomas A. Ciulla, MD, MBA, FASRS
Intravitreal bevacizumab (1.25 mg) was administered intravitreally. When she returned 8 weeks later, her macular edema resolved and NV regressed.
Condition/keywords: intravitreal bevacizumab, proliferative diabetic retinopathy (PDR)
